(Image credit: Dana Berry/SkyWorks Digital Inc.)
The Entire Universe Inside You… Your The Inner Earth.
The six most common elements of life on Earth (including more than 97 percent of the mass of a human body) are carbon, hydrogen, nitrogen, oxygen, sulphur and phosphorus. Those same elements are abundant at the center of our Milky Way galaxy. Although humans share most elements with the stars, the proportions of those elements differ between humans and stars. For example, humans are about 65 percent oxygen by mass, whereas, oxygen makes up less than 1 percent of all elements measured in space
(such as in the spectra of stars).
The proportion of each element of life differed depending on the region of the galaxy in which it was found.
For example, the sun resides on the outskirts of one of the Milky Way’s spiral arms. Stars on the outskirts of the galaxy have fewer heavy elements required for life’s building blocks, such as oxygen, than those in more central regions of the galaxy.
“It’s a great human-interest story that we are now able to map the abundance of all of the major elements found in the human body across hundreds of thousands of stars in our Milky Way,” Jennifer Johnson, the science team chair of the SDSS-III APOGEE survey and a professor at The Ohio State University, said in the statement.
“This allows us to place constraints on when and where in our galaxy life had the required elements to evolve,
a sort of ‘temporal galactic habitable zone.'”
The periodic table of elements contains all of the known elements in the universe. In this blog post,
we’ll discuss the most common elements in the universe versus the most common elements on Earth.
Naturally Occurring Elements
If you had to guess, how many elements on the periodic table would you say are naturally occurring, meaning they can be found in nature and they weren’t formed by a synthetic process? Is it all of them? Is it everything from hydrogen up to iron? We’ll get to the answer after we discuss the most common elements in the universe as a whole compared to the most common elements on Earth.
The Universe
The universe is an enormous place and is theorized to be growing larger every second. When you look up into the night sky, what are the two most prominent things you see? Stars and darkness! If you know what you’re looking for you might identify a planet or two, but it’s obvious that stars outnumber planets significantly.
In fact, of all the things visible in the universe, stars are the most common objects.
Stars are giant masses of plasma, which is superheated gas. The key word in that sentence is ‘gas.’
Since stars are the most common objects in the universe, and they are giant spheres of gas, the elements that dominate the composition of stars must be gases.
Universe Elements
If you look at the periodic table, there are only 11 elements that are gaseous.
Of those 11, two of them make up the majority of the star: hydrogen and helium. This means the most common elements in the universe are hydrogen and helium.
These two elements comprise around 98 percent of all the elements in the universe, with hydrogen at 75% and helium at 23%. Hydrogen is the simplest element that exists because it only has one proton and one electron. Every element that exists started with hydrogen.
The next three elements on the periodic table (lithium, beryllium, and boron) are over 107 times less abundant. When dealing with the universe you have to deal with unfathomable numbers!
There is a significant uptick in abundance of the next few elements: carbon, nitrogen and oxygen, but they are roughly 103 to 104 times less abundant than hydrogen. Looking at the periodic table from oxygen on, there is a steep decline in abundance of the remaining elements.
The ten most abundant elements in the universe
(in decreasing order of abundance are:)
Hydrogen
Helium
Oxygen
Carbon
Neon
Nitrogen
Magnesium
Silicon
Iron
Sulfur
In this blog post, you should have learned how the abundance of elements on Earth
compares to the abundance of elements inside the human body. A brief explanation of where the elements that can be found in the body and on Earth. The larger the star, the heavier the element it can make. The largest stars can make all elements from helium up to iron. Earth contains all of the elements generated by stars, but it is predominantly composed of a few.
Diamonds Are Forever
When we die, there are typically two options for our remains: burial or cremation. But did you know that there’s also a third option? Having your remains turned into diamonds. That’s right, when you pass on, your loved ones can wear you forever around their necks and on their fingers in the form of beautiful diamonds.
While this thought may or may not be comforting, you may also be wondering how this is even possible.
Well, due to the fact that the human body contains a large amount of the element carbon, just like diamonds,
you can absolutely be turned into a diamond when you die.
In this lesson, we’ll compare the major elements found both on Earth and in the human body.
Both the Earth and the human body contain very small amounts of elements that we won’t cover.
That’s because these elements are not major contributors to the composition of the Earth or the human body.
Elements in the Earth
Many of the elements that are abundant on the Earth are also found in the human body.
However, there are a few elements that are major contributors to the mass of the Earth that are not
significant contributors to the human body.
Iron accounts for 35% of the Earth’s mass. It’s the most abundant element on Earth.
Oxygen is responsible for 30% of the mass of the Earth. It can be found in the Earth’s crust.
Silicon makes up 15% of the Earth’s mass.
It is found in the Earth’s crust, granite, quartz, sand, and other minerals.
Magnesium accounts for 13% of the Earth’s mass. It can be found in the Earth’s crust, minerals, and oceans.
Nickel is responsible for 2.4% of the Earth’s mass. It’s found in the Earth’s core, which is made up almost exclusively of iron and nickel.
Sulfur makes up 1.9% of the Earth’s mass. It’s mostly found near volcanoes.
Calcium accounts for 1.1% of the mass of the Earth. It can be found in the Earth’s crust.
Aluminum, like calcium, is responsible for 1.1% of the Earth’s mass.
It’s also found in the Earth’s crust and minerals.
How much of the human body is made up of stardust?
Elements in the Body
Let’s look at the top elements found in the human body, beginning with those that contribute the highest percentages to the weight of the body. There are several ways to consider the composition of the human body, including the elements, type of molecule, or type of cells. Most of the human body is made up of water, H2O, with bone cells being comprised of 31% water and the lungs 83%.
Therefore, it isn’t surprising that most of a human body’s mass is oxygen. Carbon, the basic unit for organic molecules, comes in second. 96.2% of the mass of the human body is made up of just four elements:
oxygen, carbon, hydrogen, and nitrogen.
Oxygen (O) – 65% – Oxygen together with hydrogen form water, which is the primary solvent
found in the body and is used to regulate temperature and osmotic pressure. Oxygen is found in many key organic compounds.
Carbon (C) – 18.5% – Carbon has four bonding sites for other atoms, which makes it the key atom for organic chemistry. Carbon chains are used to build carbohydrates, fats, nucleic acids, and proteins. Breaking bonds with carbon is an energy source.
Hydrogen (H) – 9.5% – Hydrogen is found in water and in all organic molecules.
Nitrogen (N) – 3.2% – Nitrogen is found in proteins and in the nucleic acids
that make up the genetic code.
Calcium (Ca) – 1.5% – Calcium is the most abundant mineral in the body. It’s used as a structural material in bones, but it is essential for protein regulation and muscle contraction.
Phosphorus (P) – 1.0% – Phosphorus is found in the molecule ATP,
which is the primary energy carrier in cells. It’s also found in bone.
Potassium (K) – 0.4% – Potassium is an important electrolyte. It’s used to transmit nerve impulses and heartbeat regulation.
Sodium (Na) – 0.2% – Sodium is an important electrolyte. Like potassium, it is used for nerve signaling. Sodium is one of the electrolytes that helps regulate the amount of
water in the body.
Chlorine (Cl) – 0.2% – Chlorine is an important negatively-charged ion (anion)
used to maintain fluid balance.
Magnesium (Mg) – 0.1% – Magnesium is involved in over 300 metabolic reactions.
It’s used to build the structure of muscles and bones and is an important cofactor in enzymatic reactions.
Sulfur (S) – 0.04% – Two amino acids include sulfur. The bonds sulfur forms help give proteins the shape they need to perform their functions.2
Many other elements may be found in extremely small quantities (less than 0.01%).
For example, the human body often contains trace amounts of thorium, uranium, samarium, tungsten, beryllium, and radium. Trace elements considered essential in humans include zinc, selenium, nickel, chromium, manganese, cobalt, and lead.
Not all of the elements found within the body are essential for life. Some are considered contaminants that appear to do no harm but serve no known function. Examples include cesium and titanium. Others are actively toxic, including mercury, cadmium, and radioactive elements. Arsenic is considered to be toxic to humans, but serves a function in other mammals (goats, rats, hamsters) in trace amounts. Aluminum is interesting because it is the third most common element in the Earth’s crust, but its role in the human body is unknown. While fluorine is used by plants to produce protective toxins and has “apparent beneficial intake” in humans.
You may also wish to view the elemental composition of an average human body by mass.
The image above provides the details. For more see: Nucleosynthesis and Data Visualisation
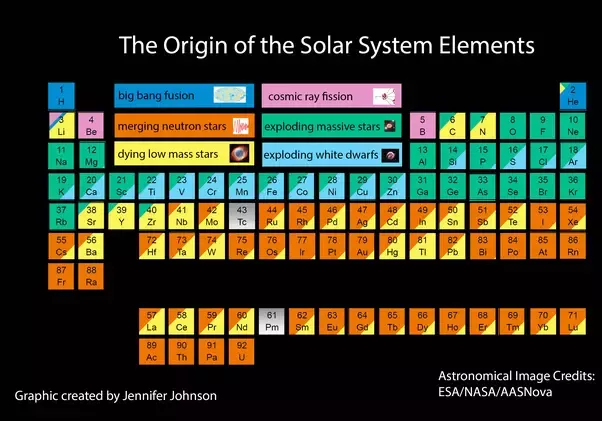
There’s only three ways to make elements heavier than hydrogen – through fusion in stars (Nucleosynthesis, a process described first by Fred Hoyle), through supernovae (aforementioned stars exploding) and neutron stars colliding (where the neutron stars are the products of the aforementioned supernovae).
So the carbon in you is a result of particles streaming away from the star (solar wind) or the outer shell of the star being ejected as the star dies or possibly lighter elements being blasted into space in a supernova.
The elements heavier than iron in you all formed from supernovae or neutron star collisions. These formed huge gas clouds in a stellar nursery. Some condensed into stars, pulling additional material into an accretion disk. This disk formed the planets and asteroids. Some asteroids (and fragments thereof) land on the Earth. The Earth accumulates a huge amount every second. In addition, the solar winds bring further elements,
just as they rip some away. So the Earth is made of star stuff of varying ages and varying pathways,
from a range of stars. And eventually some of that star stuff evolved into you.
So you are many different forms of star stuff.
Are humans actually stardust
As best as we mere mortals can put together a timeline, post Big Bang, trillions of stars were formed in the fragile cradle of the early universe. These stars lived out their lives, and then eventually exploded, hurling and producing a continuous supply of larger more complex atoms out into space. Carbon, Oxygen, Nitrogen, along with heavy metallic elements, iron, calcium, potassium, copper, for example, were created billions of years ago in the unimaginable, superheated nuclear furnace which was at the core of those earlier stars .
The primordial earth was made of that stardust, somewhere around 4 billion years ago.
The atoms of iron in your blood today, the calcium and phosphorus in your teeth, and bones all originated there. In those stars, and the eventual primordial cloud, which formed in what was ultimately to be the Milky Way galaxy. Thereafter, every organism that has ever existed on this planet, was, and is made of that “star dust”. Interestingly, of the 94 natural elements on the earth, all living organisms on this planet are composed essentially of only four of those elements, with trace amounts of metals.
So? From where did these elements come? From the countless stars that lived and died, before the Solar System came into being. And now? You and I, and all living things, past and present, on this earth, are composed of the vestiges, and byproducts, of all that nuclear productivity. We are mobile clumps, ( humans ) of stardust, that are in the process of taking in more of, and giving off more, of the stardust in different configurations, during what we call metabolism. Then other clumps are created, (born) from other clumps, some cease functioning, and return to the earth, from which more clumps absorb (eat )
and produce a continuous process of stardust borrowing .
Yes, after all is said and done? We are nothing more than stardust.
If we understand our spiritual dimension to our creation we know that the body is made from dust and the soul has a spiritual reality that is everlasting. Stars originally had hydrogen and little else. It took a star dying before all the essential elements were created in truly unbelievably huge explosions. A star blew itself up, and from that cloud of star stuff, a solar system took shape. Nobody knows how many times it had to happen before there was a good supply of material needed for Earth-building and life.
Every molecule, every atom, everything you can see around you came out of some star exploding,
producing all the elements of everyday life.
Cancer and low body temperature.
Can cancer be caused when the elements in your body become imbalanced?
“It is said in the Old Testament, “And the Lord God formed man of the dust of the ground, and breathed into his nostrils the breath of life; and man became a living soul.”[Gen. 2:7] Observe that it is said that Adam came into existence from the Spirit of life. Moreover, the expression which John uses in regard to the disciples proves that they also are from the Heavenly Father. Hence it is evident that the holy reality, meaning the real existence of every great man, comes from God and owes its being to the breath of the Holy Spirit.”
In the beginning there was but a single energy which called itself God, then one day God decided to experience something other than just being a single energy so reasoned that he/she had to be in more than one place at a time and thus decided to split himself/herself into an infinite number of pieces and each piece was a soul with the free will to think, be, say and do anything without the fear of being punished or the expectation of being rewarded. And so God was able to be everywhere at the same time and was able to experience whatever each soul experienced anywhere, as some souls choose to be minerals,
some chose to be vegetables and some chose to be animals.
Why is this question relevant? We are made of atoms….so is everything else.
Why not ask if stars are made of humans?
Amazing, Isn’t it?
Related Questions
Are we all made of stardust?
What is your opinion about the theory how humans are made from stardust?
Why has it been said that humans are made of stardust? What evidence is there for this?
Are we humans made from “stardust” and if so, how is this?
What do people mean when they say we are essentially stardust?
When someone says we are all made of stardust, do they mean this literally?
If so, how is this possible?
What is stardust? How and where was it made?
Why do scientists believe that all living things are made of stardust?
Where do souls and spirits come from if humans and other living things are made of stardust?
It is scientifically proven that we are made of stardust,
so is it possible that we become stars (light) when we die?
https://www.youtube.com/watch?v=_f2I_PoTGLU
